LAVA®
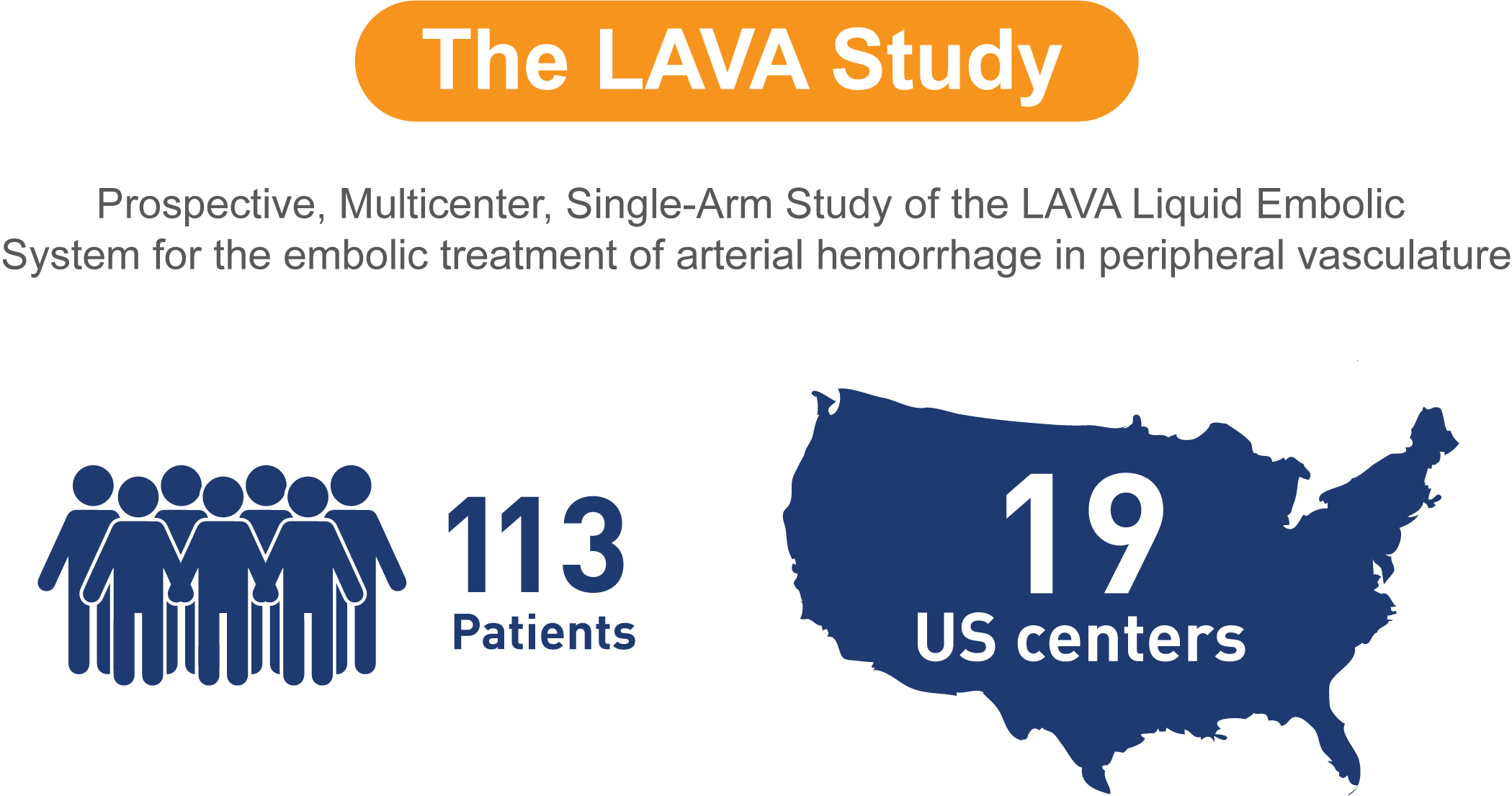
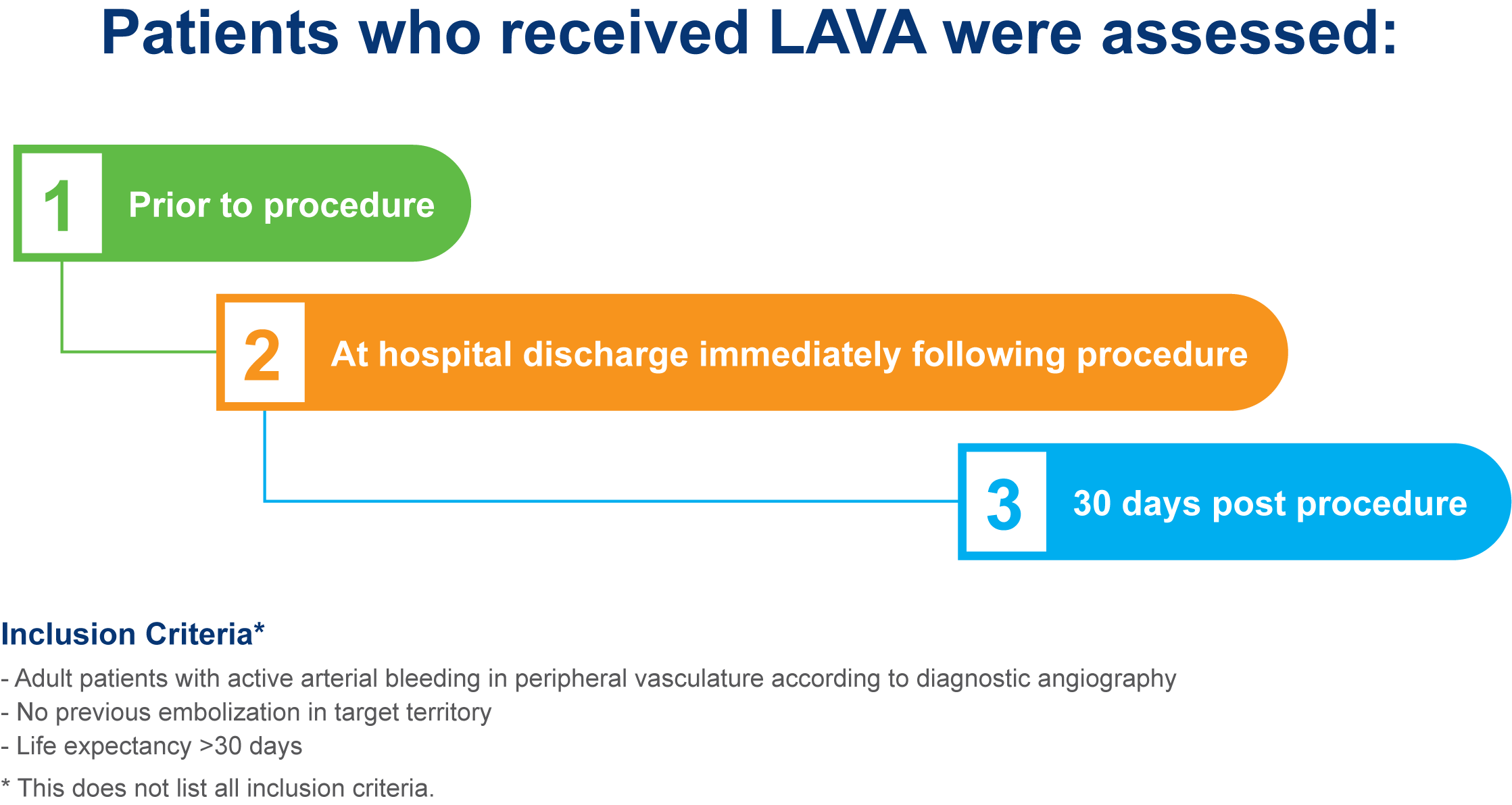
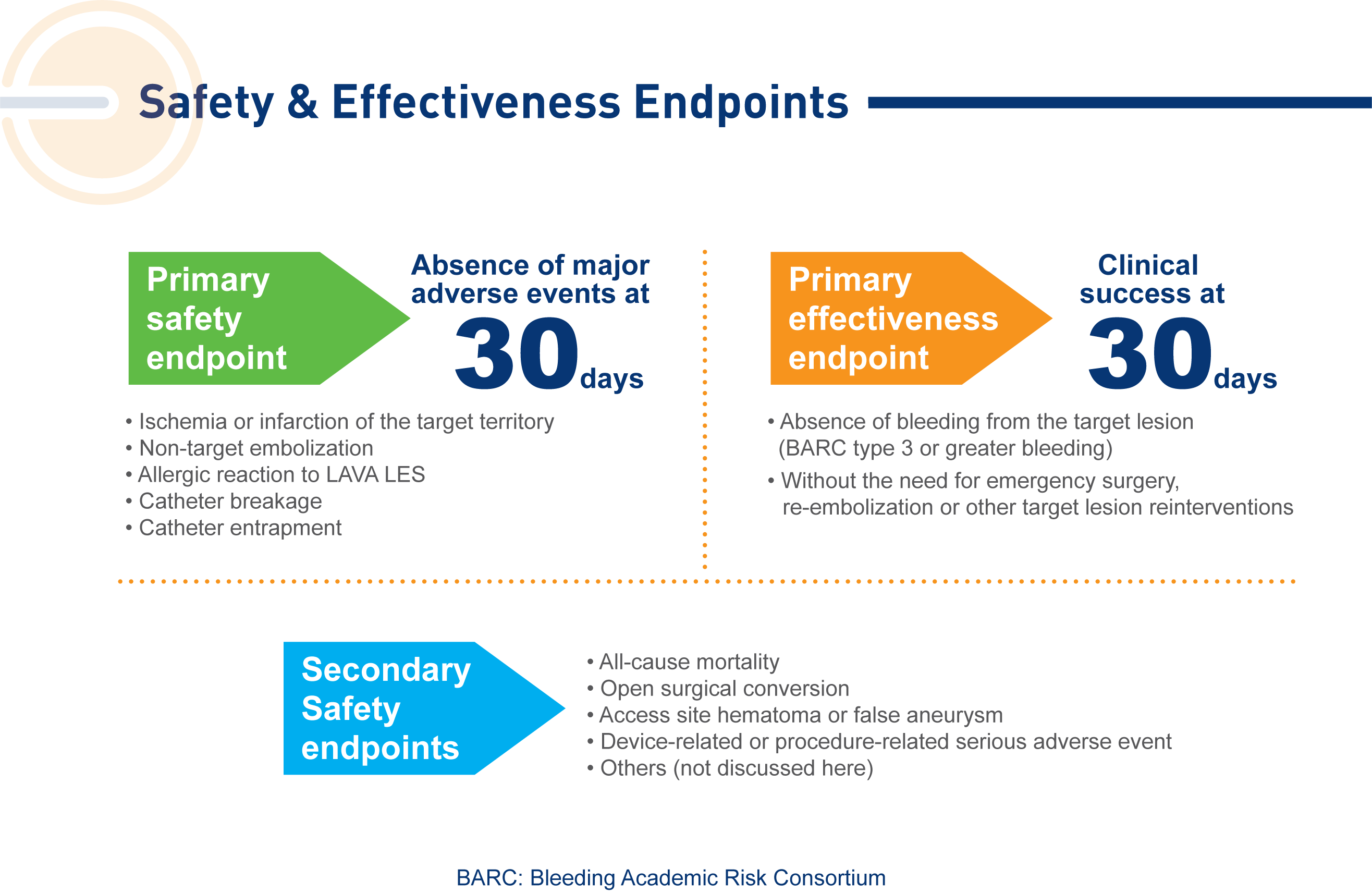
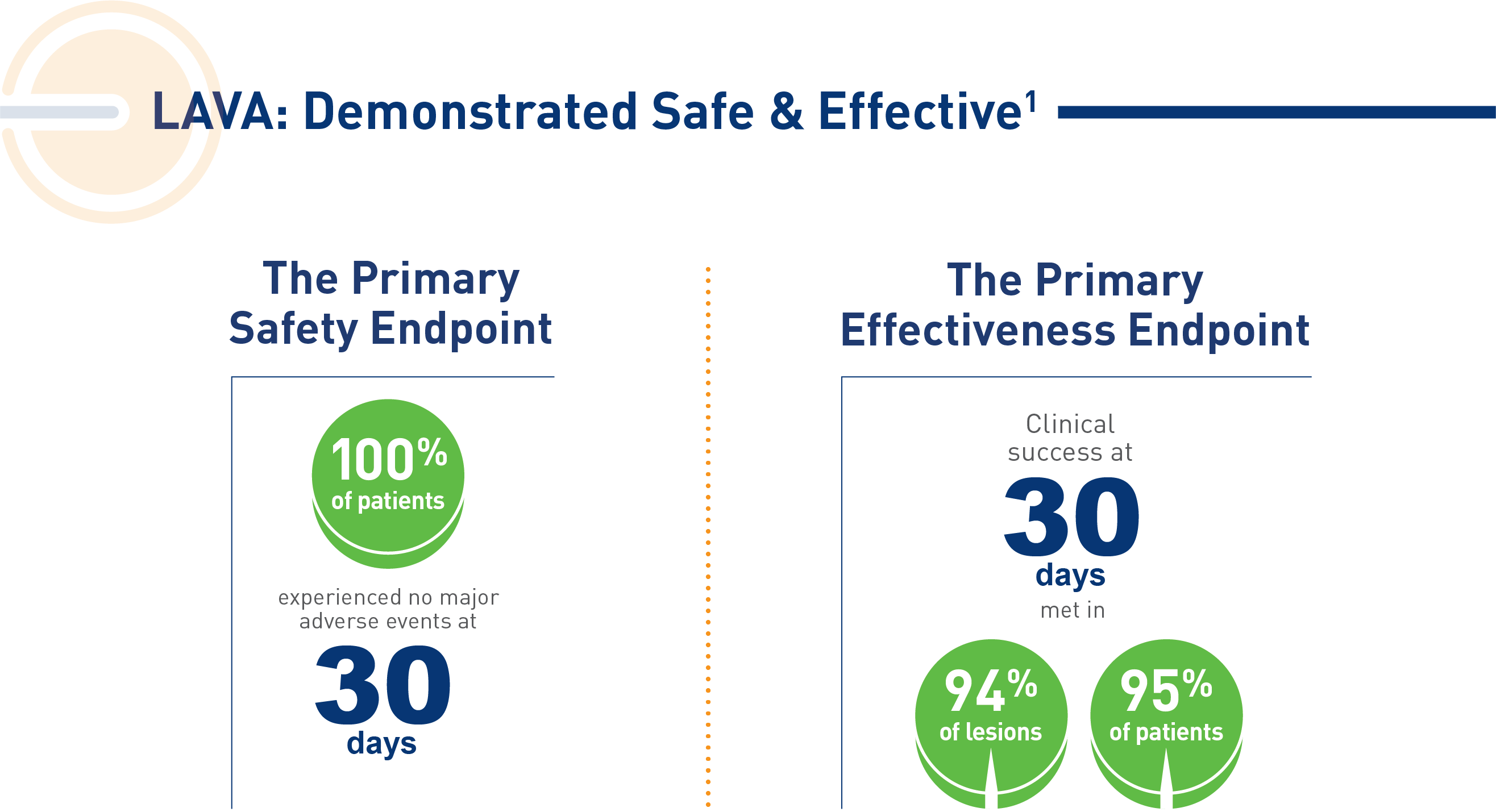
Enrolled population [n=113];
57.4(18)
Enrolled population (N=90):
8 (8.9)
Enrolled population (N=113):
63.7 (72)
use, n (%)
Enrolled population (N=96):
9 (9.4)
Enrolled population (N=113):
Hypertension - 66 (58.4)
Hyperlipidemia - 36 (31.9)
Renal insufficiency - 32 (28.3)
Diabetes - 28 (24.8)
Coronary artery disease - 21 (18.6)
Smoking - 22 (19.5)
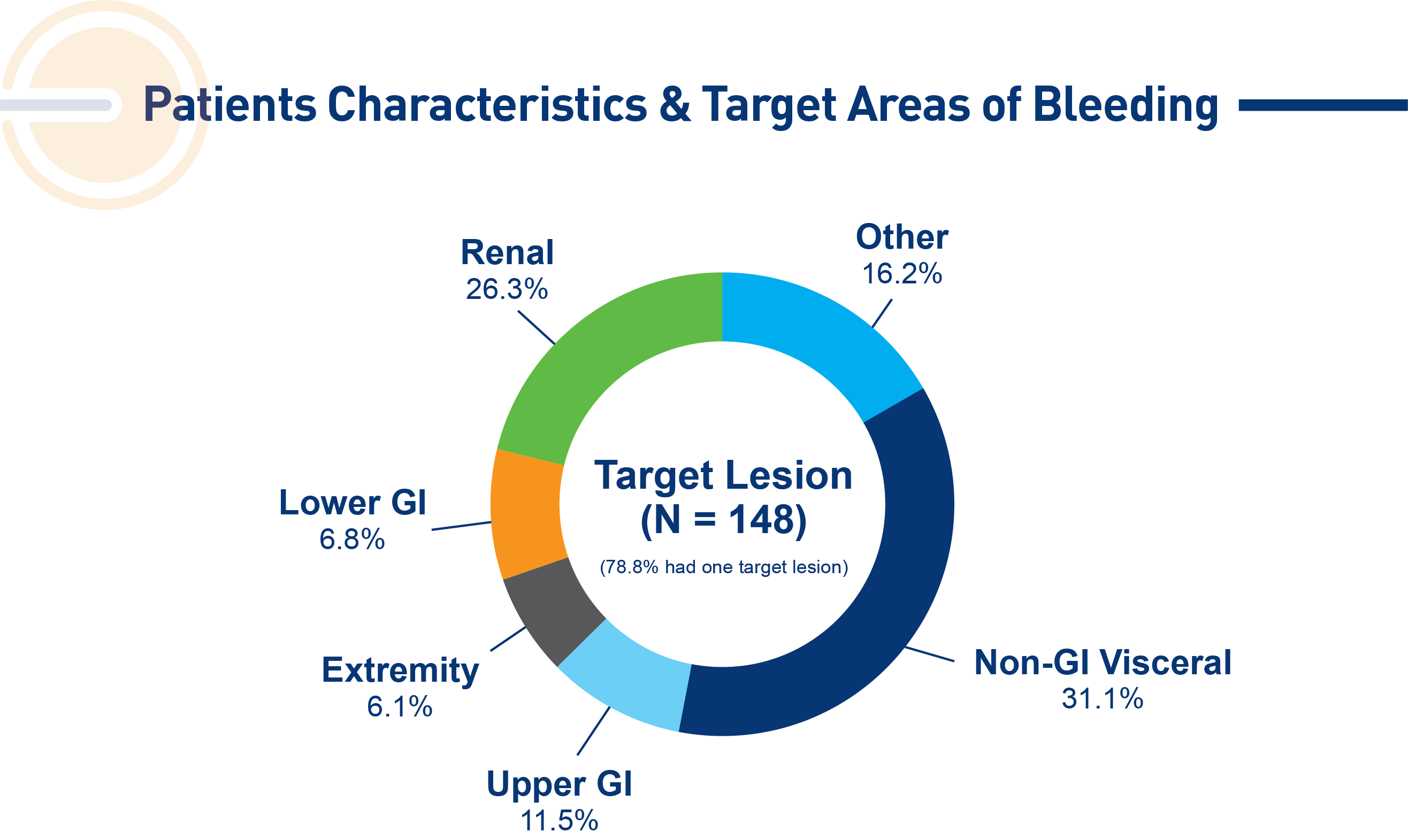
Targeted lesion population (N=148 lesions):
LAVA alone 80 (70.7)
LAVA + Coil - 23 (20.6)
LAVA + Plug - 3 (2.7)
LAVA + Gelfoam - 5 (4.4)
LAVA + Coils + Gelfoam - 2 (1.8)
for administration, min
Enrolled population (N=113):
5.9 (1-70)
1. Arslan B, et al. J Vasc Intervent Radiol, 2024.