SIR-Spheres® Y-90 resin microspheres
Procedure 2: Administration
The SIR-Spheres® Y-90 resin microspheres will be administered during a second procedure which can be conducted either the same day, the next day or one or two weeks after the initial angiogram is completed.
Before your treatment, you will be asked to come to the hospital with an empty stomach. Food and beverages are not permitted beforehand.
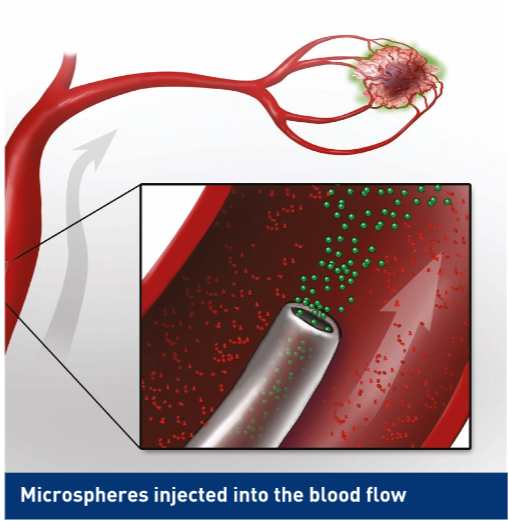
- As during the work-up, a catheter is threaded into the liver through either the femoral or radial artery.
- SIR-Spheres are injected through the catheter.
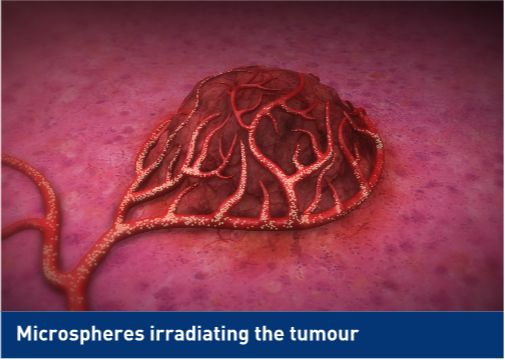
-
After the treatment
- The radiation will be half as strong after 64 hours and almost gone after eleven days.
- Immediately following the procedure, you may be taken for a scan to confirm that the SIR-Spheres have been infused into your liver.
- You will be monitored for a few hours after the procedure and discharged usually within the next one to two days (depending on the local regulations).
- Most patients can resume their normal daily activities two to three days after the treatment.
These precautions include:
- Thorough washing of your hands after going to the toilet.
- Ensuring that bodily fluids such as urine or stool are disposed of in the toilet.
You may be provided further information on these precautions by your treatment team. After two weeks, only 3% of the initial radiation remains, and after one month, it all has disappeared.
Caution: Federal (USA) law restricts this device to sale by or on the order of a physician Indications for Use: SIR-Spheres® Y-90 resin microspheres are indicated for the local tumor control of unresectable hepatocellular carcinoma (HCC) in patients with no macrovascular invasion, Child Pugh-A cirrhosis, well-compensated liver function, and good performance status. They are also indicated for the treatment of unresectable metastatic liver tumors from primary colorectal cancer with adjuvant intra-hepatic artery chemotherapy (IHAC) of FUDR (Floxuridine). Side Effects: Common side effects are fever, transient decrease of hemoglobin, mild to moderate abnormality of liver function tests, abdominal pain, nausea, vomiting, and diarrhea. Potential serious effects due to exposure to high radiation include acute pancreatitis, radiation pneumonitis, acute gastritis, acute cholecystitis and radioembolization induced liver disease (REILD). Consult the Instructions for Use for a complete listing of indications, contraindications, side effects, warnings, and precautions.